Stryker Rejuvenate Hip Recall
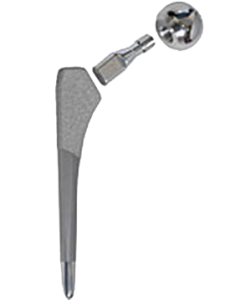 In June of 2012, artificial hip implant manufacturer Stryker Orthopaedics voluntarily recalled its Rejuvenate and ABG II Modular-Neck Stems. The Rejuvenate and ABG II hips stems differed from traditional hip systems in that the femoral component was made of two pieces, not one (known as a modular design). This was thought to be beneficial because it allowed surgeons numerous sizing and fitting options to more precisely match a patient’s anatomy.
In June of 2012, artificial hip implant manufacturer Stryker Orthopaedics voluntarily recalled its Rejuvenate and ABG II Modular-Neck Stems. The Rejuvenate and ABG II hips stems differed from traditional hip systems in that the femoral component was made of two pieces, not one (known as a modular design). This was thought to be beneficial because it allowed surgeons numerous sizing and fitting options to more precisely match a patient’s anatomy.
However, these implants failed catastrophically. The problem occurs at the metal on metal interface between the modular components, which connect to each other by a Morse taper junction. These connection points can move back and forth against each other, a process known as micro-motion. This micro-motion causes friction, which causes corrosion and fretting.
Corrosion and fretting are problematic because these processes release tiny particles of metal from the implant. These small particles, known as ions, are hazardous to patients’ health and can cause a litany of injuries, including premature device wear and failure, as well as severe adverse local tissue reactions, which can ultimately lead to further surgery to remove the implant and repair the damaged tissue.
Thousands of lawsuits have been filed against manufacturer Stryker Orthopaedics. These cases have been consolidated into a federal litigation centralized in Minnesota, as well as coordinated state court actions, including here in New Jersey, where Stryker subsidiary Howmedica Osteonics is located.
Keefe Law Firm has been deeply involved in this litigation from the outset. We represent dozens of victims who have suffered serious and permanent injuries from having a failed Rejuvenate or ABG II hip implant. In recognition of his experience and expertise in this area of law, Keefe Law Firm was appointed to the Plaintiff’s Science Committee for the consolidated litigation in the State of New Jersey.
On November 3, 2014, Stryker agreed to resolve these claims. The settlement is valued at approximately $1 billion dollars. Pursuant to the terms of the settlement agreement [link], patients who qualify and underwent a revision surgery to remove the implant are entitled to a base award payment of $300,000. Patients may be entitled to additional compensation depending upon various factors including additional injuries, treatment or surgeries, dislocations, out of pocket expenses and lost wages.
Keefe Law Firm continues to help victims of failed Stryker Rejuvenate and ABG II hip implants. If you or a loved one has been injured by a Stryker Rejuvenate or ABG II hip implant, you may be entitled to compensation. These claims are time sensitive, so please contact us today.